The depression in freezing point observed for this is 00205C. A weak acid dissociates completely at infinite dilutionSoacetic acid is a weak acid and its dissociation increases with increase in dilutionHenceacetic acid dissociates into two ionsIts dissociation constant becomes 100So by applying dissociation colligative law i 1 x 2 2 Hence vant Hoff factor for acetic acid is two.

Solved For Which Of The Following Substances The Ideal Chegg Com
Its going maybe you know 5.

. We can solve the equation for the molality of the solution. I is the vant Hoff factor. Well then why isnt it I two.
Acetic acid dimerises in benzene solutionThe vant Hoff factor for the dimerisation of acetic acid is 08. Notice that i is a property of the solute. The vant Hoff factor i is the number of particles formed in a solution from one formula unit of solute.
The Van t Hoff factor i is a measure of the effect of a solute on colligative properties such as osmotic pressure relative lowering in vapor pressure boiling-point elevation and freezing-point depression. Calculate vant Hoff factor and the dissociation constant for the acid. And so our Van Hoff factor might be something like 151 point one.
While at time t when the association is completed 1 mole of acetic acid is present. The percentage of dimerization of Acetic acid will be. 2CH 3 COOH CH 3 COOH 2.
2 moles of acetic acid associate to form 1 mole of dimer so that. ΔT b is the boiling point elevation. α 50 1 2 given Substitute the value of α 50 1 2 and n in above formula we have.
Kb is the boiling point elevation constant. But Vant Hoff factor 052 thus 1-x 2 052 1-052 x 2 x 096 degree of association of acetic acid 96 Note. Kf for cyclohexane is 200 degree per molal and density of acetic acid at 200 degrees celcius is 1049 gml b Calculate the molecular mass of acetic acid in cyclohexane.
I 1 1 1 2 α 1 α 2. Acetic acid dimerises in Benzene solution. A a ΔT b iKbma a.
In an ideal solution i does not depend on the concentration of the solution. Freezing point of solution cyclohexane acetic acid. If the solute is a nonelectrolyte ie.
For example sucroses sucrose. We know the formula of vant Hoff factor which is given as. The vant Hoff factor for an ionic compound is the number of ions that form when the compound dissociates.
I 1 α α n. Um que its less than one. The vant Hoff factor for a non-ionizing molecule is 1.
21 degrees Celcius a Calculate the vant Hoff factor for acetic acid CH3COOHl in cyclohexane C6H12l. So n 2. Why is the vant Hoff factor for solutions of acetic acid CHCOOH slightly greater than 1 but far short of i 2.
Displaystyle i1-left 1- frac 1 2rightalpha 1- frac alpha 2 For association in the absence of dissociation the. So its possible to pick any volume of solution to determine. Northern cod produces proteins that protect their cells from damage caused by subzero temperatures.
T TiKci Kc nnn n nnn 32 32 32 32 CH CO H original CH CO H original H CH CO H original H CH CO H original tion and equilibrium. It does not separate into ions in solution i 1. It is a weak electrolyte.
The vant Hoff factor for materials which are soluble in non-polar solvents is typically 1 because these things are typically non-ionizing molecules. 1-x 2 The total number of particles at equilibrium is equal to the Vant Hoff factor. 06 mL of acetic acid CH 3 COOH having density 106 gmL is dissolved in 1 litre of water.
Here two mole of acetic acid produce 1 mole of dimer acetic acid. This reaction isnt going 100. The of dimerisation of acetic acid is.
The Van t Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and the concentration of a substance as calculated. Acetic acid CH2COOH is an organic compound present in vinegar. In the question acetic acid in benzene is mentioned so here degree of association comes into account.
The K value for this reaction from equilibrium. As we know Vant Hoff Factor i fractextObserved colligative propertytextNormal or theatrical colligative property On keeping the values - i frac12 05. A Acetic Acid C2H4O2 b Sodium dichromate Na2Cr2O7 c Magnesium bicarbonate Mg HCO32 d Toluene C-Hg Input an integer in the each answer box.
The formula for boiling point elevation is. Thus at t0 when association did not start 2 moles of acetic acid is present. A III 80180 Mark for Review 0115 hr mir vant Hoff factor of acetic acid which shows 10 dimerization in water is 095 075 080 090 Clear Response Answer N2 dimerisation B001 1 1 8 6 -1 i 2-1x 1²-1 X-1 1 x 1 -12 - 1 05 i 95 i.
The Vant Hoff factor for the dimerization of Acetic acid is 08. And the reason is because this reaction doesnt really happen that much. Determine the value of vant Hoff factor i for the following compounds.
A student performed an experiment and calculated the vant Hoff factor value for acetic acid to be 186 at 20C. 102 For 01 mol 0002 mol Percen n nn nn n total acetic acid 0002 H tage ionization 100 100 2 ionized 01 V C V. M is the molality of the solution.

Ncert Solutions Ncertsolutionsforclass12 Ncertsolutionsforclass12chemistry Ncertsolutionsforclass12chemistrychapter2soluti In 2021 Chemistry Solutions Dissociation

The Van T Hoff Factor Of Acetic Acid Solution Is Than One And The Value Of Normal Colligative Property Is Than The Observed Colligative Property Of This Solution

Van T Hoff Factor For Aqueous Monofluorouroacetic Acid Is Youtube

Solved Van T Hoff Factor Of Acetic Acid Is Right And What Chegg Com

Finding The Van T Hoff Factor Youtube

The Van T Hoff Factor Of Acetic Acid Solution Is Than One And The Value Of Normal Colligative Property Is Than The Observed Colligative Property Of This Solution
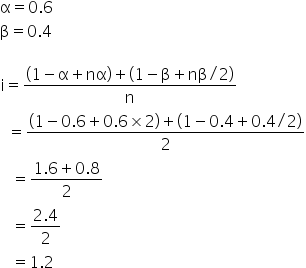
Value Of Van 39 T Hoff Factor If Ch3cooh 60 Dissociates And 40 Dimerized Is 1 12 2 14 3 16 4 18 Chemistry Topperlearning Com Mzkruvii

Imp Osmosis Passive And Active Transport Types Paysage Fantastique

The Van T Hoff Factor Definition And How To Calculate It Physical Chemistry Osmotic Pressure Positive Numbers
0 Comments